ORDI announces the RACEFOR7 2023 program, scheduled this year in 13 Major Cities in India with an expectation of over 20000 participants in the event. More than 10000 people participated in Racefor7 2020, the last physical event. In 2021 and 2022, ORDI organized virtual running events which had over 4000 participants. This year over 20000 participants are expected […]
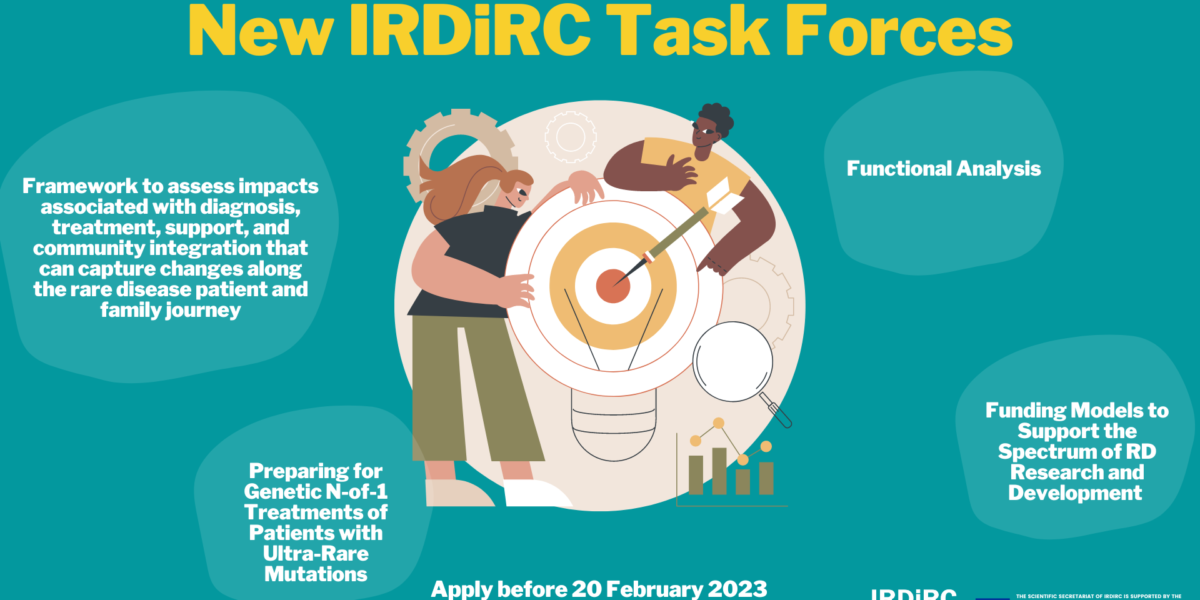
Read MoreIRDiRC launches the Call for Members for the four new Task Forces of the 2023 Roadmap. Check out the newly approved Task Forces: Funding Models to Support the Spectrum of Rare Disease Research and Development The overall objective of this Task Force is to identify how different types of funders make decisions about when to […]
Read MoreThe National Institutes of Health (NIH)’s National Center for Advancing Translational Sciences (NCATS) will co-sponsor this year’s Rare Disease Day (RDD) at NIH event with the NIH Clinical Center. This event aims to raise awareness about rare diseases, the people they affect, and NIH research collaborations that address scientific challenges and advance research for new […]
Read MoreOn 15 December 2022, Orphadata Science was awarded Global Core Biodata Resource status, being one of the first batch of resource to be designated by the Global Biodata Coalition. Orphadata Science (which includes Orphanet‘s scientific knowledge base) was one of the 12 European resources to have been selected following a two-step application process evaluated by […]
Read MoreAFM Téléthon (France) launches a scientific call for proposals for 2023, open both to French and foreign teams. The aim is to support: Fundamental Research and Physiopathology of Diseases of the Neuromuscular System Development of Innovative Therapeutic Approaches for Rare Genetic Diseases More information available here (PDF) Along with this call, AFM Téléthon also launches […]
Read MoreJoin us on 8th December for the European Health Summit that will take place in Brussels (Belgium) and online to find out more about our journey towards a Rare Disease Moonshot. Online registration is open here: https://lnkd.in/eCGr3ZmX IRDiRC Scientific Secretariat Coordinator, Daria Julkowska, will be part of the panel “Building a sustainable R&D ecosystem for […]
Read MoreIn the context of the IRDiRC Interview Series, Marjon Pasmooij’s interview is now available. Marjon is a member of IRDiRC’s Therapies Scientific Committee and Science Programme Manager at Medicines Evaluation Board (CBG/MEB), University Medical Center Groningen, The Netherlands. Watch the full interview here
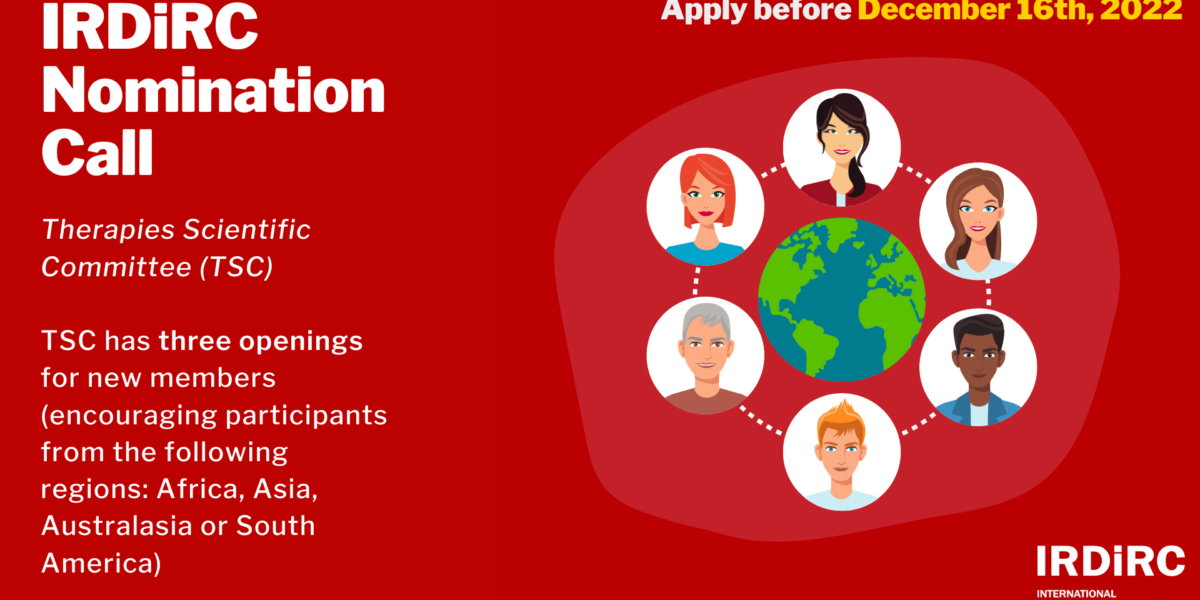
Read MoreIRDiRC has four Scientific Committees, one each for Diagnostics, Therapies, Interdisciplinary, and Regulatory aspects of rare diseases research. The Therapies Scientific Committee (TSC) is a multi-stakeholder, multi-disciplinary group of experts in medical research and therapy development in rare diseases. Specifically, the TSC is devoted to pursuing the therapeutic development of IRDiRC, supporting the rare diseases […]
Read MoreThe registration for the Third International Summit on Human Genome Editing is now open. The event will take place on 6-8 March 2023 at the Francis Crick Institute, London UK. Register for free here The Third International Summit on Human Genome Editing will take place on 6-8 March 2023 at the Francis Crick Institute, London […]
Read MoreIn addition to the Orphan Products Grants Program that the Office of Orphan Products Development (OOPD) currently administers, a new grant program was established this year by the Accelerating Access to Critical Therapies for Amyotrophic Lateral Sclerosis Act (ACT for ALS) named the FDA Rare Neurodegenerative Disease Grant Program. This new program will be administered […]
Read More